Background: Ibrutinib (IBR) inhibits CLL proliferation and effects prolonged remission without eradicating disease. Obinutuzumab (OBI) is an anti-CD20 monoclonal antibody that can effect depletion of measurable residual disease (MRD) below 0.01%. In the IcICLLe study (ISRCTN12695354), 20 treatment-naïve (TN) CLL patients & 20 with relapsed/refractory (R/R) CLL received IBR until complete remission with <0.01% MRD in the bone marrow (BM) or disease progression. The IcICLLe Extension Study examined the efficacy/safety of combining IBR & OBI in 40 patients with R/R CLL and was open to the IcICLLe R/R cohort. Initial results after 1 month of combination treatment indicated that adding OBI to IBR improved CLL depletion, and 3-5 year follow-up data is now available.
Aim: To assess the long-term MRD response status for patients treated with IBR as a sole agent or in combination with OBI.
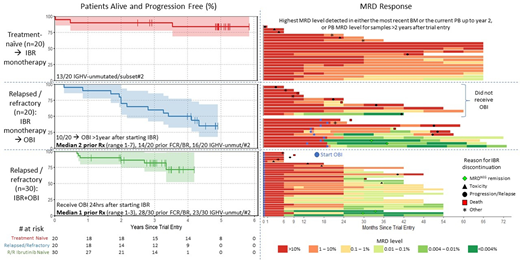
Patients: Patients received continuous IBR (420mg OD) with the addition of 6 cycles of OBI given over 6 months in the extension study. 20 TN patients received IBR monotherapy; 20 R/R patients (median 2 prior treatments, range 1-7) initiated IBR monotherapy of which 10/20 enrolled in the Extension study receiving OBI after >1year of IBR monotherapy; and 30 IBR-naïve R/R (median 1 prior treatment, range 1-3) started OBI 24 hours after first IBR dose. MRD assessment was performed according to ERIC guidelines with a maximum detection limit of 0.001%/10-5.
Results: IBR monotherapy in TN patients was well tolerated with 18/20 patients alive and 13/20 remaining on IBR after median 4.9 years follow-up (range 0-5.9). IBR was stopped due to toxicity (3), progression/relapse (2) or other causes (2). IBR-monotherapy resulted in median 0.65 log depletion per year in years 1-2, followed by relatively stable disease levels (median 0.2 log depletion per year) in the subsequent 3 years in 13/20 evaluable patients. Only 1 patient showed >0.3log increase in PB MRD level and this preceded clinical progression. No patients achieved an IWCLL CR/CRi and MRD was >0.01% in PB/BM in all patients at all time points.
In the R/R group initially receiving IBR-monotherapy, 11/20 patients remain alive and 3/20 remain on IBR after a median 3.9 years follow-up (range 0.3-5.3). In this heavily pre-treated group, 10 did not enrol on the OBI Extension study (1 died, 1 progression/relapse, 4 ineligible/other causes, 4 patient preference) with 10/20 receiving OBI at median 16.2 months (range 13-19) after starting IBR-monotherapy of which 7/10 had resolved any lymphadenopathy pre-OBI. 2/10 achieved MRD-negative remission and stopped treatment, while 6/10 have since stopped IBR due to death (2), progression/relapse (3) or other causes (1). 30 R/R patients with no prior IBR exposure (most in first relapse) started IBR & OBI at the same time: 26/30 remain alive and 18/30 remain on IBR after median 3.0 years follow-up (range 0.8-4.2). IBR was stopped in 2/30 patients achieving MRD-negative remission while 10 stopped IBR due to death (2), disease progression/transformation (3), toxicity (4), or patient decision (1).
There were no Grade 5 adverse events related to OBI. 3 months post-OBI, patients with >1 year prior IBR-monotherapy achieved a higher response rate (CR/CRi 50% vs. 30%), MRD response (<0.01% BM MRD in 50% vs. 6%) and a greater depth of remission (3.1 vs. 1.5 log reduction) compared to IBR-naive patients despite greater number of prior treatment lines.
The deepest PB MRD responses were observed 6-12 months after the last OBI infusion. MRD levels showed little change in the following year to month 24 (median 0 log depletion, range 1.3 log depletion to 2.5 log increase). After 2 years of IBR exposure, the MRD levels shows a similar pattern to TN patients on IBR monotherapy, being generally very stable (<0.3log increase) except in 7 patients showing >0.3log increase at two sequential timepoints of which 4/7 have shown clinical disease progression and 3/7 still have low (<1%) but rising MRD levels.
Conclusions: The addition of OBI to IBR may substantially improve depletion of CLL cells from the PB and BM. A greater impact in MRD response rate and depth was seen when OBI was introduced after >1 year of IBR treatment and tumour bulk was low. One fifth of patients maintain <0.01% MRD up to 3 years after combined IBR+OBI, demonstrating that response improvements associated with OBI are sustainable in some cases.
Rawstron:Abbvie: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Pharmacyclics: Consultancy, Honoraria, Research Funding; Roche: Honoraria, Research Funding. Hillmen:AbbVie: Speakers Bureau; Janssen: Speakers Bureau; Gilead: Speakers Bureau; Pharmacyclics: Other: Financial or Material spport; Morphosys: Other: Consulting fees; Sunesis: Other: Consulting fees. Brock:AstraZeneca: Current equity holder in publicly-traded company; GlaxoSmithKline: Current equity holder in publicly-traded company; Eli Lilly: Other: Consulting and speaker fees; Invex: Other: Consulting and speaker fees; Merck: Other: Reimbursement of costs. Patten:Roche: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astra Zeneca: Honoraria; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Pettitt:Chugai: Research Funding; Gilead: Honoraria, Other: Hospitality, Research Funding; Kite: Honoraria, Other: Hospitality, Research Funding; Celgene: Other: Hospitality, Research Funding; GSK: Research Funding; Novartis: Research Funding; Napp: Research Funding; Roche: Honoraria, Other: Hospitality, Research Funding. Fox:Abbvie: Honoraria, Research Funding; Adienne: Honoraria, Research Funding; AstraZeneca: Research Funding; Celgene: Research Funding; Sunesis: Research Funding; Atarabio: Research Funding; Roche: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Gilead: Honoraria, Research Funding. Bloor:Abbvie: Consultancy, Honoraria, Other: Conference Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Other: Conference Funding , Speakers Bureau. Hillmen:Apellis: Consultancy, Research Funding, Speakers Bureau; Gilead: Other: Financial or material support, Research Funding; AstraZeneca: Consultancy, Speakers Bureau; Acerta: Other: Financial or material support; Roche: Consultancy, Other: Financial or material support, Research Funding, Speakers Bureau; AbbVie: Consultancy, Other: Financial or material support, Research Funding, Speakers Bureau; Pharmacyclics: Other: Financial or material support, Research Funding; Janssen: Consultancy, Other: Financial or material support, Research Funding, Speakers Bureau; Alexion: Consultancy, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal